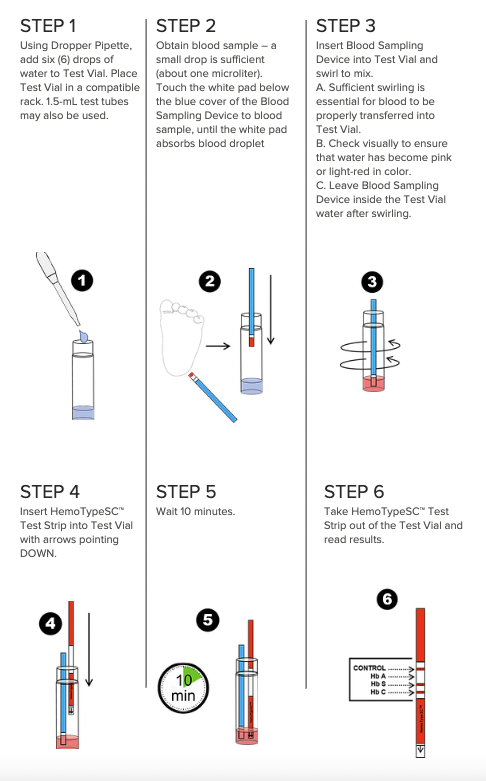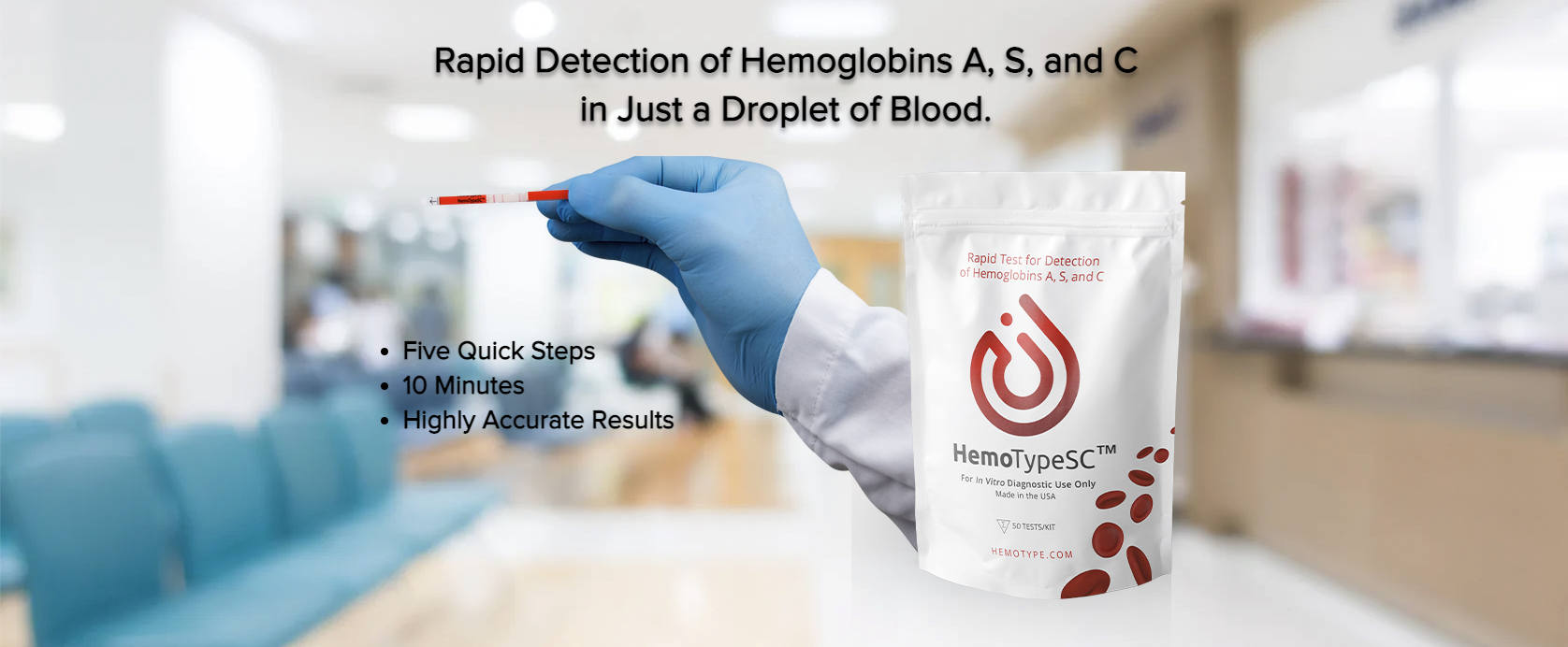
How It Works
Success Stories
Global Registrations

Peer-Reviewed Publications
Côte D’Ivoire, 2021
Feasibility Study of the “HemoTypeSC” Test for the Rapid Screening of Sickle Cell Disease in Côte D’Ivoire
Danho, et al.
D.R. Congo, 2020
Acceptability of neonatal screening of the sickle cell disease during the pandemic of COVID-19 in Kisangani, Democratic Republic of the Congo
Kasai, et al.
Nigeria, 2020
HemoTypeSC point-of-care testing shows high sensitivity with alkaline cellulose acetate hemoglobin electrophoresis for screening hemoglobin SS and SC genotypes
Adegoke, et al.
Nigeria, 2020
Implementing newborn screening for sickle cell disease as part of immunisation programmes in Nigeria: a feasibility study
Nnodu, et al.
Nigeria, 2020
Feasibility and acceptability of early infant screening for sickle cell disease in Lagos, Nigeria-A pilot study
Oluwole, et al.
Tanzania, 2020
Extremely high birth prevalence of sickle cell disease in rural Tanzania
Eastburg, et al.
India, 2020
Multicenter Evaluation of HemoTypeSC as a Point-of-Care Sickle Cell Disease Rapid Diagnostic Test for Newborns and Adults Across India
Mukherjee, et al.
Uganda, 2019
HemoTypeSC Demonstrates >99% Field Accuracy in a Sickle Cell Disease Screening Initiative in Children of Southeastern Uganda
Nankanja, et al.
Nigeria, 2019
HemoTypeSC, a low-cost point-of-care testing device for sickle cell disease: Promises and challenges
Nnodu, et al.
Ghana, 2019
Point-of-care screening for sickle cell disease in low-resource settings: A multi-center evaluation of HemoTypeSC, a novel rapid test
Steele, et al.
USA, 2016
A rapid, inexpensive and disposable point-of-care blood test for sickle cell disease using novel, highly specific monoclonal antibodies
Quinn, et al.
In The News

N.Y.A.N.
amsterdamnews.com
New Sickle Cell Disease Testing on Nigerian Newborns
A newly released study seeks to increase sickle cell disease detection and treatment rates in newborn babies across Nigeria through HemoTypeSC, a rapid diagnostic test.
Quartz Africa
qz.com
A 10-minute Test Could Solve Africa’s Sickle Cell Disease Screening Problem for Newborns
A $2, 10-minute rapid point-of-care tool has brought the much elusive goal of rapidly screening Africa’s newborns for sickle cell disease (SCD), a major cause of child mortality, within reach.
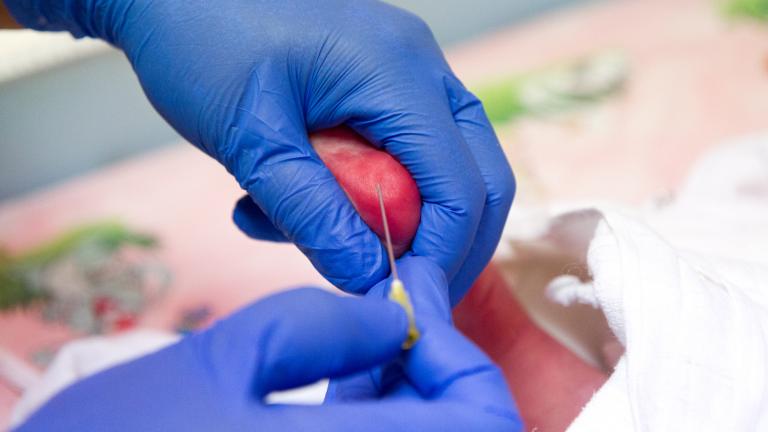
NIH/NHLBI
nhlbi.nih.gov
Rapid Result Test On Track to Transform Sickle Cell Disease Screening for Millions
Now, research shows that with a new rapid result test kit, a diagnosis of sickle cell disease may no longer be a death sentence for children in the most affected parts of the world…The test has the potential to be a game-changer for people with the disease who live in distressed areas around the world.

Medpage Today
medpagetoday.com
$2 Test Could Transform Sickle Cell Dx in Africa
An inexpensive, easy-to-use blood test could dramatically alter how sickle cell disease is diagnosed in Africa, where the often-undiagnosed disease is a leading cause of childhood mortality.

Medscape
medscape.com
New Sickle Cell Test Can Transform Screening
A new test that costs less than $2, requires only a small drop of blood, and can be performed in about 10 minutes could dramatically change the dynamics of sickle cell diagnosis in Africa.

CISION
prnewswire.com
New Tools and Approaches Poised to Improve Diagnostics and Care for Millions Worldwide
A trial in Uganda showed a new test called HemoTypeSC was more than 99 percent accurate in detecting sickle cell disease in young children. The test requires only a small drop of blood and returns results in about 10 minutes, making it suitable for routine screening of newborns.

Journal de Brazza
journaldebrazza.com
Advocacy for the Construction of a Sickle Cell Anemia Care Center in Côte d’Ivoire
Patrice Sékongo, country director of the European Institute for Cooperation and Development, called for mass screening and improved patient care thanks to a preventive system that is operational. In a presentation on the evolution of screening tools, Dr Yao Atimeré, a hematologist, presented the HemoTypeSC test, which can detect sickle cell anemia in ten minutes. A solution that comes to save lives.

Fraternité Matin
(Newspaper Article)
Good News for Ivorian People. Now, They Can Know Their Hemoglobin Status Thanks to the HemoTypeSC Test…
Eugène Aka-Aouélé, Minister of Health and Dr. ADOUENI Katché Valéry, Coordinating Director of the National Program for Metabolic Diseases and NCD Prevention, have stated that the [HemoTypeSC test] will enable Ivory Coast to take a step forward in the fight against sickle cell disease.
Radiodiffusion
Télévision ivoirienne
Television News Coverage of Accuracy+Feasibility Validation Study Workshop – Côte d’Ivoire
The Nation
thenationonlineeng.net
Lack of Funds Hinders Govt’s Policy on SCD
Speaking at the presentation of HemoTypeSC, a care test for the detection of SCD, Dr. Alayo Sopekan, National Desk Officer on Sickle cell, Ministry of Health, [noted] that the country has put in place a policy to address the disease, such as testing for newborn, Sopekan, however, said the lack of fund has not allowed for the implementation.
SilverbirdTV
silverbirdtv.com
Experts Advocate Newborn Screening for Sickle Cell Disease
Professor Obiageli Nnodu, sickle cell disease expert from the University of Abuja, describes the current status of sickle cell disease in Nigeria and the results from early testing with HemoTypeSC™.
Frequently Asked Questions
What Does HemoTypeSC Detect?
HemoTypeSC detects hemoglobin A, hemoglobin S, and hemoglobin C using highly specific monoclonal antibodies. Therefore, six different hemoglobin phenotypes can be resolved: HbAA, HbAS, HbAC, HbSS, HbSC, and HbCC.
HemoTypeSC is blind to fetal hemoglobin, meaning that even samples with very high HbF (i.e. from newborns with >95% HbF content) can be accurately screened.
Has HemoTypeSC Been Approved for Use as an in Vitro Diagnostic?
Yes, HemoTypeSChas received CE Mark approval as an IVD and is registered as an IVD in many countries. See the “Registrations” section of this website for more information.
How Does HemoTypeSC Work?
HemoTypeSC is a competitive lateral flow immunoassay. The hemoglobin in the blood sample “competes away” the corresponding test line on the test strip. Therefore, a missing line means a positive result for that particular hemoglobin.
How Long Does the Test Take?
Ten minutes.
Can HemoTypeSC Be Used for Newborns? How about Adults?
HemoTypeSC may be used to screen individuals of all ages and is ideal for screening newborns. Each monoclonal antibody in HemoTypeSC is highly sensitive for its respective hemoglobin and is blind to fetal hemoglobin. So, even newborns with very high levels of fetal hemoglobin and low levels of other hemoglobins may be confidently screened.
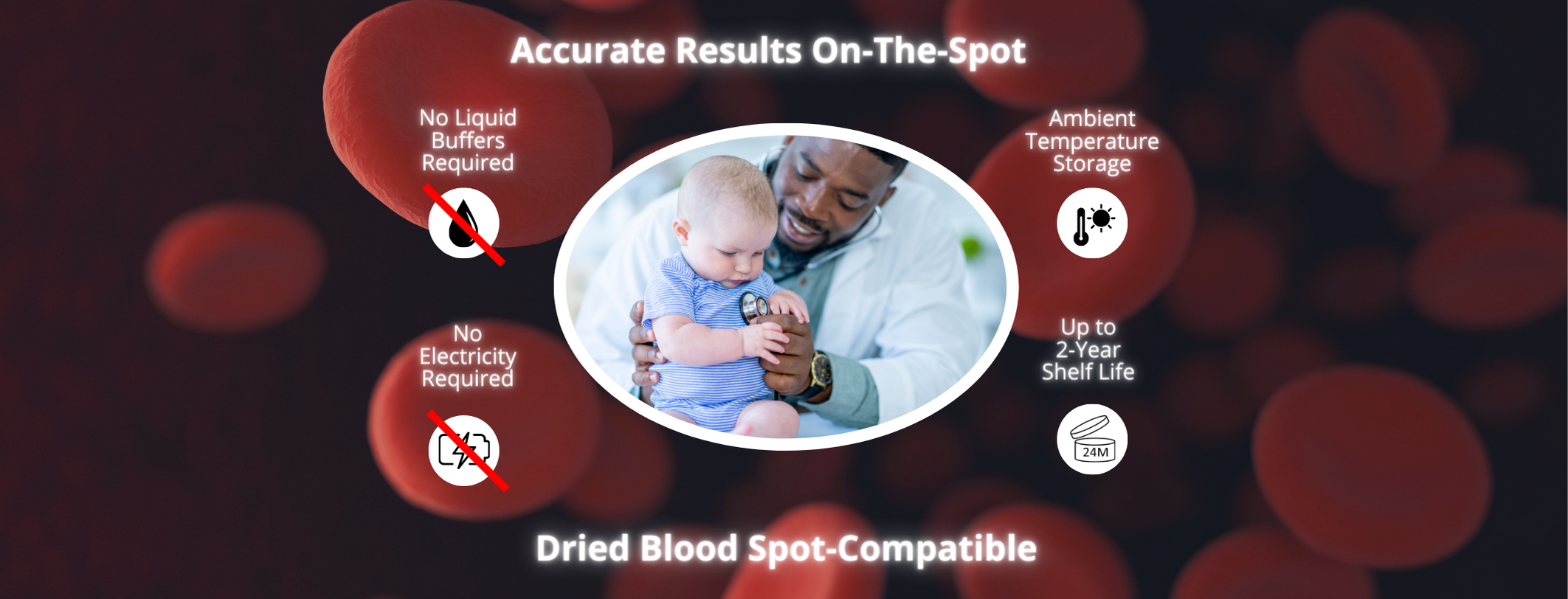
Can Dried Blood Spots Be Tested?
Yes! HemoTypeSC is unique in that we have validated a very simple protocol for screening dried blood spots (DBS) in addition to fresh whole blood. Please contact us for the DBS screening protocol.
What Is the Shelf Life?
Two years from the date of manufacture.
How to Store the Product?
HemoTypeSC should be stored away from moisture at room temperature (between 15 and 40 degrees Celsius).
What Do I Need in Order to Run a HemoTypeSC Test?
Each HemoTypeSC test kit includes test strips, blood transfer devices, and water transfer pipettes. Clean test tubes or vials with a rack and a small amount of clean, fresh water are additionally needed for the test but not included with the test kit.
How Accurate Is HemoTypeSC?
HemoTypeSC has repeatedly displayed >99% sensitivity >99% specificity in over one dozen independent field validation studies conducted throughout Africa, India, and the Caribbean, involving thousands of patients.
How Can I Purchase HemoTypeSC?
Please contact [email protected] for details about procuring the test in your particular country.
Have Other Questions?
Contact us at [email protected]
Our Distributors

Distributor for India
CareAbility Healthcare LLP.
A-208, 2nd Floor, Somdatt Chamber-1, 5 Bikaji Kama Place, New Delhi- 110066. Delhi, India.
E-mail: [email protected]
Website: www.careability.co.in
Email us by submitting a message using the form below!